STUDY SKILLS
The factual style of writing in science textbooks is different from the narrative style of most other books you read. Thus, reading science requires special skills. In order to be successful in chemistry, you must adjust to the amount of factual information presented. You must read critically and sense relationships among ideas, as well as build on previous knowledge. You must master a new, technical vocabulary. Skills are needed to use supplementary materials, like this problems book, to enhance understand
ing. You must learn to interpret information presented in tables, graphs, and diagrams. Laboratory experimentation also requires special skills to interpret and apply scientific knowledge. In this chapter you will learn some techniques that can help you master these skills.
1:1 READING SCIENTIFIC MATERIALS
Reading for meaning, or comprehension, is one of the most important skills to master. Once the foundation is laid for strong comprehension the other skill areas will be facilitated. For example, if you have a basic understanding of the concepts presented in a particular chapter, new vocabulary words will be grasped more quickly from context clues. Problems and laboratory activities will also gain perspective.
When previewing or reading you should identify the main ideas in the material. The main idea is the most important concept described in a passage. The main idea can be located at any point in a paragraph and may not be a single sentence. The main ideas in a sequence of paragraphs can serve as a summary of the material.
You should always try to understand what you read and avoid rote memorization. One way to broaden your understanding is to look for statements that support the main idea. Some of these statements may be real world examples of the main idea; others may be conclusive research data. Supporting statements often explain the "how" or "why" questions you may have about the main idea. You should associate these supporting statements with the main idea. These associations will help you increase your knowledge from factual repetition of main ideas to broader understanding of the concept described.
One additional type of statement you should look for as you read is linking sentences. Linking sentences provide perspective on the material. The linking sentences tie the main idea to previously learned material or to material to be presented later. Use linking sentences to determine where to store new information in your mind. As you add new information to it various compartments of information your knowledge base will expand. You will also avoid trying to remember lots of unrelated concepts. Let’s practice some of these suggestions with a paragraph from your textbook.
Carbon atoms may bond to each other by the overlap of an orbital of one carbon atom with an orbital of another carbon atom. The carbon-carbon single bond is a sigma type bond. Many carbon atoms may bond in this manner to form a chain or ring. Recall from Chapter 11 that carbon exhibits catenation. Plastics, synthetic fibers, and synthetic rubber all contain molecules with hundreds or thousands of carbon atoms joined in chains and rings.
The main idea is italicized. This information alone is not very helpful to our general understanding of carbon bonding. However, if you remember that carbon atoms may bond by orbital overlap and they bond in chains or rings, two "how" questions are answered. The fourth sentence serves as a link with previous information. The last sentence provides real world application of the main idea.
1:2 DRAWING CONCLUSIONS
You may be asked to draw conclusions concerning a passage you have read. The answer to the question may not be stated directly in the passage. However, by understanding the main idea and some supporting details you can make an educated guess. For example, you know that pressure is caused by the collisions of particles with the walls of their container. You also know that temperature increases the speed of particles. You conclude that pressure increases with an increase in temperature because the particles would have more collisions. When you make conclusions you may use inductive reasoning. Inductive reasoning involves applying specific concepts to general situations to form a conclusion. For example, consider the following.
Hans Geiger and Ernest Marsden subjected a very thin sheet of gold foil to a stream of subatomic particles. They found that most of the particles passed right through the sheet. From this observation Rutherford concluded that the atom is mostly empty space. They also found that a few particles (about 1 in 8000) bounced back in almost the opposite direction from which they started. Rutherford explained this observation as meaning that there was a very small "core" to the atom. The core contained all the positive charge and almost all the mass of the atom. This core is now called the nucleus.
1:2 Drawing Conclusions
Rutherford used inductive reasoning to develop this theory of atomic structure. He used specific data from the Geiger-Marsden experiment to develop a general description of atomic structure. Deductive reasoning involves applying general concepts to answer specific problems. Following is an example of how deductive reasoning is used.
Every system has some internal energy which is designated by U. The internal energy is a state function. Since we will be interested only in ~U, changes in the internal energy of a system, we do not have to know absolute values of U for systems.
General information about the change in internal energy is used to find specific answers to the problems in Chapter 20.
Read the following paragraph and answer the questions that follow. The density of gases and vapors is most often expressed in grams per cubic decimeter. We express it in these units because the usual density units, g/cm3, lead to very small numbers for gases. It is possible to calculate the density of a gas at any temperature and pressure from data collected at any other temperature and pressure. Assuming that the number of particles remains the same, a decrease in temperature would decrease the volume and increase the density. An increase of pressure would decrease the volume and increase the density. The following problem illustrates this calculation. (Remember 1000 cm3 equals 1 din3.)
1. The purpose of this passage is to describe
a. how many cm are in 1 dm3
b. the units used for gas density
c. typical density units
d. the effects of temperature and pressure on gas density
2. In what units is gas density measured?
a. g/cm3
b. 1000 cm3
c. g/dm3
d. 1 dm
3. What value must remain constant in order to determine gas density?
a. number of particles
b. temperature
c. pressure
d. volume
4. Which of the following is not mentioned in the paragraph?
a. effects of temperature
b. ffects of pressure decrease
c. usual density units decrease
d. gas density units
5. What can you expect to learn in the text that would follow this paragraph?
a. effects of temperature on volume
b. effects of pressure on volume
c. how to calculate gas density
d. conversion units for density
6. Compare gas density units with usual density units. You would conclude that
a. gases are less dense than other forms of matter
b. gases are more dense than other forms of matter
c. gases have the same density as other forms of matter
7. In order to answer question 6 you must use
a. deductive reasoning
b. inductive reasoning
8. If the temperature of a sample of gas changed from 520C to 230C, the volume of the gas ....would
a. increase
b. decrease
c. remain the same
9. In order to answer question 8 you must use
a. deductive reasoning
b. inductive reasoning
c. remain the same
1:3 GRAPHING
Throughout your study of chemistry you will be required to prepare graphs or interpret graphs of experimental data. Thus you should be familiar with the composition of a graph.
The data that you will be using involves two variables, dependent and independent. The quantity that is deliberately varied is the independent variable. The quantity that changes due to variation in the independent variable is the dependent variable. The independent variable is plotted on the horizontal axis. This axis is referred to as the abscissa or x axis. The dependent variable is plotted on the vertical axis. The vertical axis is referred to as the ordinate or y axis.
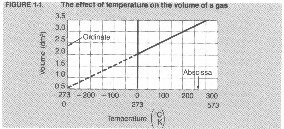
Graph titles should clearly state the purpose of the graph and include the dependent and independent variables For example, Figure 1-1 is titled
FIGURE 1-1. 
Chemistry Skills 5
volume vs. temperature of a gas. Each axis should be labeled with the appropriate variable and units of measurement. For Figure 1-1 the abscissa is labeled Temperature (K, C0), indicating that the scale is marked in both kelvins and Celsius degrees. The ordinate is labeled Volume (din3), with the scale marked in cubic decimeters. Each axis has equal intervals. The intervals are 0.5 din3 for the ordinate and 1000C or K for the abscissa.
1:4 INTERPRETING GRAPHS
Sometimes it is necessary to find a value for a variable at a point along the graph that is not one of the original data points. For example, on Figure 1-1 assume that we want to know the volume of a gas when the temperature is 750C. The original data was not recorded at 750C. In order to determine the volume of the gas at 750C you must interpolate, or read from the graph between data points. By interpolating we can see that the volume of the gas is 2.3 din3 at 750C.
If a value is needed that is beyond the limits of the graph you must extrapolate. Reading a graph beyond the limits of the experimentally determined data points is extrapolation. To determine the theoretical volume of a gas at —2730C we must extrapolate to find the answer, 0 din’. You should be cautious when extrapolating data. The relationship between the variables may not remain the same beyond the limits of your investigation.
CHAPTER REVIEW PROBLEMS
Read the following paragraph and answer the questions.
An immediate application of electrochemistry is its use in quantitative analysis. For example, suppose that it is necessary for you to determine the percentage of copper in a given water soluble copper compound. A sample of the compound of known mass could be dissolved in water and inert electrodes inserted. The mass of the cathode should be measured before the current is applied. As the current is passed through the cell, metallic copper plates onto the electrode of known mass. When the action is complete and current no longer flows, all the copper is plated. The mass of the electrode is again measured. The difference in mass (due to the copper) is compared to the mass of the sample to find the percentage of copper in the sample. Electroanalysis is a useful tool of the chemist.
1. The best title for this passage is
a. Electroanalysis
b. Electrochemistry
c. Copper Analysis
d. Chemistry Tools
2. All the copper has been plated when
a. the copper compound dissolves in water
b. the percentage of copper in the sample is determined
c. current no longer flows
d. the mass of the electrode is measured
3. Which of the following is not mentioned as a step in plating copper?
a.compound is dissolved in water a sample of copper
b. the electrodes are attached to a power source
c. the mass of the cathode is measured
d. electrodes are inserted into the copper solution
4. With what topic is this passage most closely associated?
a. finding mass of substances
b. electricity
c. electrochemistry
d. chemistry of copper
5. Electroanalysis is
a. an application of electrochemistry
b. a useful tool of the chemist
c. a form of quantitative analysis
d. all of the above
6. You should conclude that the
a. increases with plating
b. decreases with plating
FIGURE 1-2.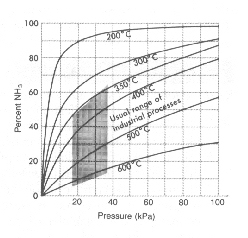
What is the title of the graph?
What variable is plotted along the abscissa?
What variable is plotted along the ordinate?
In what units is pressure measured?
What is the interval for each block along the y axis?
At 70 kPa and 6000C, what is the percent yield of NH3?
Predict the percent yield of NH3 at 120 kPa and 2000C.
Considering the usual range of industrial processes, what is the maximum yield that can be expected?
In order to obtain at least a 90% yield, in what temperature range would NH3 be produced (pressure not to exceed 100 kPa)?
| HOME |

A Non-Profit Education Corporation suggestions or donations  |